A History of the Discovery of Element 103
|
JINR Reports (1989), Dubna, Russia.
|
During 1958-61 at Berkeley several attempts were made to synthesize element 103 by bombarding curium and californium targets by the ions of nitrogen and boron, respectively. By that time the HILAC linear accelerator was put into operation and produced beams of the above ions with reasonable intensities. The targets were made from Cm and Cf accumulated in reactors with enhanced thermal neutron fluxes. It was planned to perform the synthesis in reactions of the type (HI,xn). Their small cross sections predetermined the difficulties in the identification of the new elements detected according to their α radiation. The background from α emitters in the Po-Ra region produced in Pb and 8i impurities which could imitate the α particles due to the new element was especially troublesome. This fact was mentioned in papers by the group engaged in the synthesis of element 102 in Moscow [Ml]. Therefore the target decontamination and the choice of the method of the identification of the new element was of crucial importance. Chemical identification was not feasible because of expected very short lifetimes of the isotopes which could hopefully be synthesized. In principle, a most credible physical method is the one which permits the establishment of genetic relationship between the unknown nuclide and its daughter product characterized by well known radioactive properties by means of α-decay [2]. Ghiorso’s group used this approach in their preceding attempt to synthesize element 102 [83]. Another complementary evidence which may have independent demonstrative power under certain conditions is the established correspondence between the yield of the observed nuclide versus the projectile energy dependence and the expected excitation function of the reaction postulated. This method can certainly be used in some cases, for example, if the target is monoisotopic and the reaction is of the evaporation type, (HI,xn). These were, in our view, the material and ideological prerequisites of the experiments designed for synthesis of element 103 at Berkeley in the early sixties. The chronology of the attempts to synthesize element 103 is as follows. The first experiments date back to 1958 [B3]. A target composed of 244Cm (95%) and 246Cm (4%) was bombarded by 14N ions. The nuclear reaction products knocked out of the target and stopped in a gas were collected on a catcher by the eleotrostatic deposition of the ionized atoms. The α-radiation of the collected products was detected by photoemulsions. A weak α-activity with an energy of 9+-1 MeV and a half-life of about O.25 s was observed. It was supposed to be due to the isotope 2561O3 from the 244Cm (14N,4n)256l03 reaction. To prove this statement it was necessary to carry but additional experiments but the authors [B3] have never communicated a continuation of that study. It was possibly explained by the radiation accident which took place – the destruction of a curium target in one of the experiments [B4]. In 1960 Ghiorso [85] reported the experiments carried out to synthesize the isotope 259103. A 252Cf target about 1 mug in mass was bombarded with 11B ions. The identification of 259103 was attempted by establishing its relationship with the known nuclide 255Fm in the following chain 259103 α—>255Md EC—>255Fm by the “double recoil method” [83] (see Section 3 about element 102). 255Fm was detected on the catcher foil for daughter products and its distribution along the catcher corresponded to T1/2=0.2 sec for 259103. However control experiments showed that 255Fm could appear as a result of the “leakage” onto the collector directly from the zone of irradiation of the primary atoms of 255Md, produced in the reaction 252Cf( 11B,α4n)255Md. That followed from the fact that the 255Fm counts did not disappear when the velocity of transport of primary atoms to the zone of collection of daughter products was decreased substantially. The leakage was confirmed by the detection on the collector of 256Md produced by the reaction 252Cf(11B,α3n)256Md and observed by recording the spontaneous fission of the daughter nuclide 256Fm. All this indicated that the experimental method was not perfect. In the third attempt the Berkeley team reverted to the direct observation of α-decay of element 103 amongst reaction products. In those experiments they used surface-barrier spectrometric detectors with a resolution of 50 keV to record α-particles. In 1961 Ghiorso and coworkers published a paper [86] in which they claimed for priority in the discovery of element 103 and proposed to name it “lawrencium”. They aimed to synthesize the element in the reactions Cf(B,xn)103 by using 11B and 10B ion beams. The 3 mug target had the following isotopic composition: 3% 249Cf, 33% 250Cf, 12% 251Cf, and 51% 252Cf. Special measures were taken to remove Pb and Bi impurities from the target material. Nuclear reaction products were knocked out of the target into an atmosphere of helium, stopped there and then transported to the negatively charged tape serving as a collector. The transport was performed using the gas flowing slowly through an orifice about 1 mm in diameter. The tape was periodically pulled to place the reaction products successively in front of each of five spectrometric α detectors.
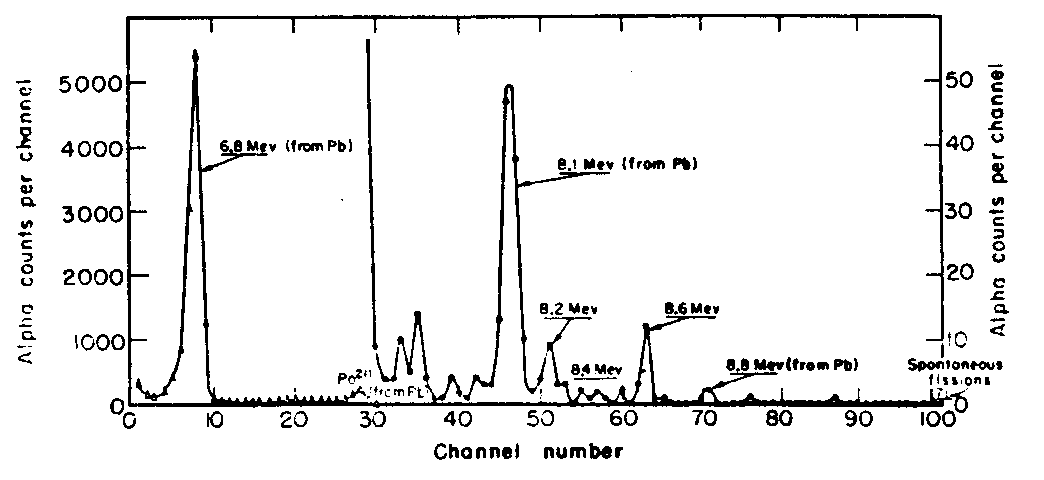
Fig.1. “Cf + 11B. α spectrum from first detector. Summation of Runs Nos. 139-148. Total bombardment = 5.0 muA hr. Cycle time = 15 seconds”. (Fig.2 from Ref. 6). The spectra shown in fig. 1 exhibit α activity with Ea=8.6 MeV. It was asserted that its half-life was measured to be equal to 8-2 s. This figure is the only concrete quantitative material presented in the article, while other results are only mentioned. The authors assigned this α activity to the nuclide 257103 on the basis of the following considerations. The given activity was not observed in bombardments of Pb, Bi and 241Am targets with 1OB and 11B ions. That the 8.6 MeV activity could not be attributed to light isotopes of mendelevium was evidenced by the fact that the activity had not been recorded in the bombardments of 241Am with 12C ions. The authors also presented some considerations regarding the mass number of the isotope emitting 8.6 MeV α particles. In their view, that was 257103 since the observed excitation functions were very broad both in the 10B and 11B ion bombardments. That might happen only in the case of formation of the nuclide sought for simultaneously in several reactions (x=3-6) involving different Cf isotopes of the target material. The possibility of assigning the 8.6 MeV α activity to isotope of element 102 from reactions of the type (B.pxn) was ruled out by the following authors’ considerations and data. The 8.2 MeV α activity with T1/2=15 s, observed in the spectrum (fig. 1), was tentatively assigned to 102. In the C bombardments of the Cf target (the bombarding energy is not given) the yield of the 8.2 MeV activity was increased by a factor of 20 while that of the 8.6 MeV activity was decreased by a factor of 2. The authors considered this to be positive proof for the assignment of the 8.6 MeV activity to an element 103 isotope since such a ratio could supposedly be expected from the experiments with 240Pu. "Experiments with Pu240 had shown that the (C12, αxn) cross sections would be larger, while 12 the (C12 ,pxn) cross sections would be smaller in comparison with the boron bombardments of californium". In 1965 the Dubna group discovered 256103 [D7] and in 1967 it was shown [D8] that 257103 had not the properties described in the Berkeley work of 1961 [B6] . Me note that in 1967 in Dubna it was shown that 102 also has properties differing from those ascribed to it in [86] (see Section 3 about element 102). Shortly after that Seaborg discussing the "257103" of [6] wrote in 1967 in his review article [89]: "it now seems more likely that it should be assigned to lawrencium-258 or -259". Finally, the 1971 paper by Eskola et al [B10] on the element 103 isotopes with A=255-260 read as follows: "In the present work it is shown that 257Lr has a half-life of 0.6 s-and the main α-particle group at 8.87 MeV. An isotopic assignment for the 8.6-MeV activity which is consistent both with the 1961 results and those presented here is 258Lr. The difference in the half-life values is due to relatively poor statistics in the former study". These two quotations are the only reaction of the Berkeley group to the fact that the conclusions made in paper [86] have not been confirmed. Making references to this re-interpretation the Berkeley authors did and still do insist on their priority in the discovery of element 103.
Immediately after the changed interpretation of work [86] by Seaborg [89] (see the above quotation) Donets et al. published review article [Dll] in which they thoroughly analyzed work [B6] itself and its revision [9] and expressed serious criticism. The analysis we have made shows that in fact still more questions arise.
It goes without saying that it is impossible to make more or less far—reaching conclusions from the mere observation of unknown (or, may be, simply unidentified) activity. Let us consider the arguments put forward in Ref. 86 to confirm the correctness of the identification of the atomic number Z in that sequence (in all likelihood, following the chronology of the experiments) in which they are given in the original paper. At first it is pointed out that, along with the activity of Ea=8.6 MeV and T1/2 =8-+2 s, the spectra also contained a particles with energies of 8.4 and 8.2 MeV and close half-lives of about 15 s. Further, in the same paper, only 8.2 MeV is discussed and it is postulated (without justification) that the activity is due to 102. As we now know, this nuclide has Ea=8.1 MeV and T1/2 =3 min, that is quite different properties. The authors [86] emphasize that "these activities have been observed repeatedly during many weeks" in both 10B and 11B bombardments. Of course, that was the necessary indication of the actual existence of the activities, but not more.
Then follows the first argument that "similar bombardments of Pb, Bi, 240Pu, and 241Am do not produce the new activities" (apparently the three activities are meant). The experimental conditions (ion energy, fluence, etc.) are not described and, therefore, it is impossible to judge the level of significance of the negative result. If we assume it to be fairly high and the ion energy to vary in the necessary range, then from the experiments with 240Pu + 10,11B it follows that the new activities could not be due to the Es isotopes with mass numbers smaller than 248 or to the products of their decay. Similarly, the 10,11B bombardments of 241Am give the limit of A<249 for Fm isotopes and their decay products. At this point it should be noted that by 1961 no one isotope of element 102 was identified correctly, and only the isotopes with A=255 and A=256 were known for Md. Thus between the indicated limits for Es and Fm isotopes and the supposedly synthesized nuclide 257103 (and, what is more, 258103 or 259103) there remained a large area of the then unknown isotopes of Md and element 102, which could not be eliminated on the basis of the bombardments of 240Pu and 241Am.
The authors believed that under the conditions of their experiments reactions might occur which could involve the fusion of Cf and B nuclei followed by the evaporation of 3 to 6 neutrons from the compound nucleus, i.e. they expected reactions of the type Cf(B,xn)l03 to occur. To illustrate this we present the x values of these reactions in table 1.
Cf (10B,xn) l03 reactions Cf (11B,xn) l03 reactions
Note that the cross sections of the reactions with x=4 and x=5 are almost equal in the maxima of the excitation functions and are by one order of magnitude larger than the maximum values for the x=3 reactions and several times larger than for the reactions involving the emission of 6 neutrons. The cross section for the reaction in 250Cf is nearly a factor of 2 smaller than that for 252Cf. Reactions with x =1 or 2 practically do not proceed; therefore they are not included in table 1. Further, the cross sections of the reactions with x=3 and x=4 reach their maxima at the same B ion energy (59 MeV) but for x=5 and x=6 the cross section maxima are displaced by several MeV to higher energies (64 and 80 MeV for 11B, 65 and 75 MeV for 10B). The width of the excitation functions at half maximum is 8-1O MeV; the verbal characterization of the excitation function width in Ref. B6 should be understood just as related to such a "standard" characterizing the narrow excitation function. If we now take into account all the indicated properties of the excitation functions and the isotopic composition of the target, we can rightfully expect the effective cross section versus ion energy dependence (the term "excitation curve" used in [86] does not seem appropriate here) to have the following character:
– the curve for 259103 should be narrow in both the 10B and 11B bombardments; 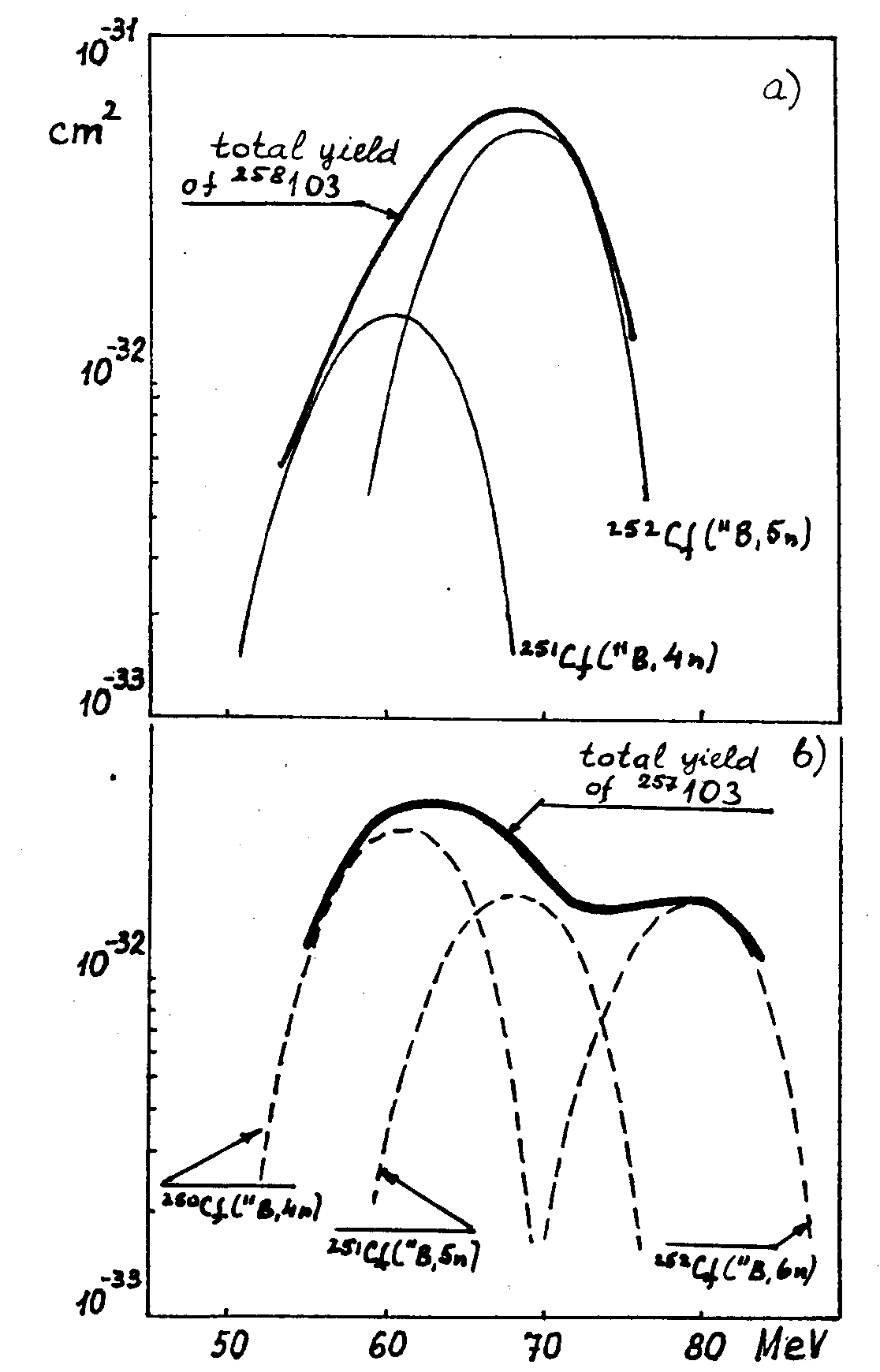
b) 257103 from the reactions 250Cf (11B,4n), 251Cf (11B,5n), 252Cf(11B,6n). There is no doubt that on the basis of exactly the same anaiysis the authors [86] assigned the new activity to 257103 after observing "very broad excitation function". The curve itself is not given in the paper and, moreover, there is no indication of the range of the ion energy within which it has been measured. Therefore it is difficult to judge why isotopes lighter than 257103 have not been considered. As for the isotopes 259103 and 258103, their excitation function should be narrow, which contradicts the experimental observations [86]. Consequently, the attempts of Seaborg [B9] and Eskola et al [B10] to re-interpret the results [B6] are unjustified and have no grounds.
After noting that the yield curve for 257103 was of necessity" very broad, the authors of [B6] conclude that the final proof Of Z = 103 requires the elimination of the possible (B,pxn) reactions At this point it looks as if it is asserted, though not explicitly, that the given quantitative ratios of the yields which were measured on a Cf target are close to the results of similar experiments with a Pu target. On the other hand, no results whatsoever are presented regarding the bombardment of 240Pu (all is restricted to the above quotation), nor were they published later.
The bombardment of 243Am with 12C ions allows one to produce Md isotopes with mass number 252 and below. Again the range of C ion energies is not indicated and the experimental sensitivity is not given in terms of cross section. As a result, the lower limit of the "verified" mass numbers is unclear. In this connection we note that 248Md very much resembles one of the three activities discussed in ref. [B6]. As is seen from the above analysis, paper [B6] practically does not contain quantitative characteristics of the experimental data and the conditions under which they have been obtained (ion energy, atom collection efficiency, the efficiency of α counting, the cross section sensitivity, etc.). Therefore, in this form it cannot be verified experimentally. Later the authors agreed that their identification of the nuclides observed has been erroneous. But, proposing to re-interpret the results [B6] in support of the discovery of 258103 [B9,B10] they have not so far made any attempt to prove the consistency of the basically new interpretation with the contents of paper [B6]. It is obvious that only just the fact that the radioactive properties of 258103 (Ea = 8.6 MeV, T1/2 = 4s.) determined later in [B10] turned out to be similar to those of the activity observed in [B6] and atrributed to 257103, cannot form the basis of the claim for the discovery of element 103. Paper [B6] does not contain more or less serious arguments in favor of the Z identification. Auxiliary bombardments did not cover the whole region of nuclides which should be ruled out. The results of the only attempt of cross bombardment cannot be the conclusive proof. The ion energy dependence of the 8.6 MeV activity yield reported without the indication of the energy range gives nothing for the Z=103 identification and excludes the possibility of assigning this activity to 258103 or 259103. Thus the reinterpretation [B10] is not "consistent with the 1961 results" (broadcurve).
3. Dubna work on the synthesis of element 103.
At Dubna the studies aimed at synthesizing element 103 were begun in 1965. Donets et al [D7] obtained the isotope 256103 in the reaction 243Am(18O,5n)256103. The identification of 256103 was made by detecting the granddaughter nuclide 252Fm which could be formed in the following chains:
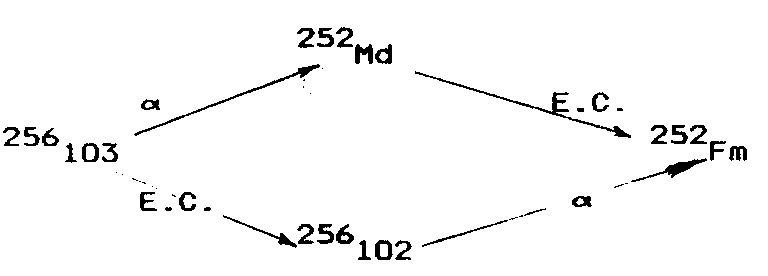 The technique provided the identification irrespective of the decay mode of 256103 (α or EC). Use was made of the same experimental apparatus (fig. 3) as that used in the synthesis of 256102 [D12]. Nuclear reaction products were knocked out of the target into an argon-filled closed volume, stopped in the gas and diffused onto the walls of a circular notch in the disc which transported than from the target region to the zone of daughter product collection. The charged recols formed as a result of α decay were carried to the surface of a collector by an electric field in the gas. Fermium, the 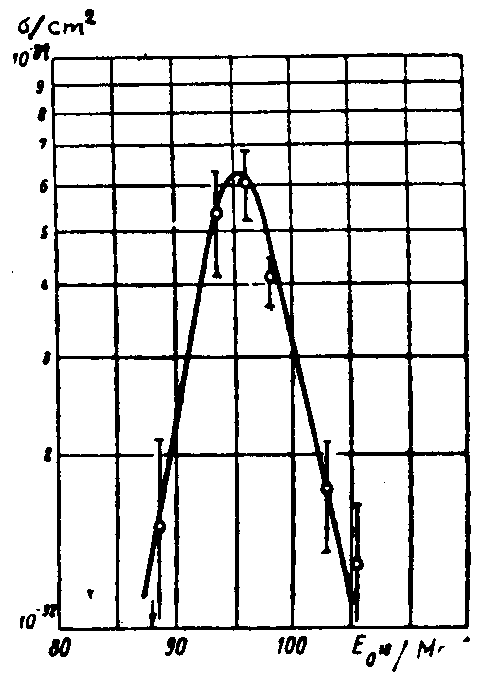
product of the radioactive decay of 256103, was chemically separated from the collector material and other actinoids by using ion-exchange methods. The α activity of the fermium fraction was measured using an α spectrometer including surface-barrier detectors.
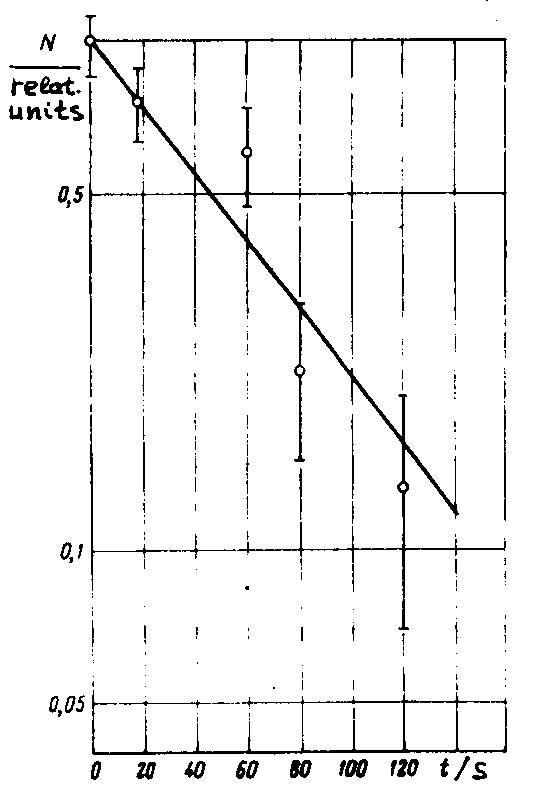
In 1967 in Dubna the experiments aimed at synthesizing isotopes of element 103 were continued. The work was carried out by two groups [D15, D16] using different experimental devices in which nuclear reaction products were collected from a gas stream. The a radiation of the products was detected directly and their identification was made according to the excitation functions of the corresponding nuclear reactions and by using cross bombardments. Much attention was paid to the thorough purification of targets from lead impurities. Fig.6 taken from [D15] shows the α spectra of the products formed in the 18O bombardments of 243Am at energies of 91 and 96 MeV. The α groups observed in the 8.35-8.6O MeV range were attributed to 256103 decay. The appearance of the 8.8 MeV group was
 Fig. 6. The α spectrum measured in the systems 243Am + 18O (a,b) and Pb + 18O (c). The total duration of the irradiation and measurement is 25 s. From Ref.D15. due to Pb impurities in the target material. The α spectrum shown in fig. 6b and obtained in the bombardments of lead, suggests that the background from lead impurities did not exceed 1O% in the experiments carried out to produce 256103, in the energy range between 8.3 and 8.6 MeV. The background was determined by normalizing to the Ea=8.8 MeV line. The half-life of the Ea=8.3-8.6 MeV group was measured to be equal to 35 s, which agreed with the 256103 data [D7]. The dependence of the activity yield upon the bombarding energy corresponded to that for the reaction 243Am(18O,5n)256103.
In 1970 in Dubna the isotope 255103 was synthesized in the reaction 243Am( 16O,4n) 255103 and its properties were determined: Ea=8.38 MeV and T1/2=2O s [D16].
Thus the 1965-1967 studies carried out in Dubna [D7,D15,D16] to synthesize 256103 resulted in the identification of this nuclide at first by investigating the granddaughter nuclide 252Fm and then its α-decay half-life was measured. The actinoid chemical character of element 103 was determined in 1968 in Dubna using frontal chromatography of gaseous chlorides (cf. section 3.3.6) with the same isotope. Somewhat later the 256103 radioactivity properties determined in Dubna were confirmed completely by the study carried out in Berkeley [B10]. Bearing in mind that the data presented in ref.[B6] had not been confirmed the authors of the 1967 Dubna work applied to IUPAC with the proposal to name element 103 "rutherfordium".
In 1971 a Berkeley team carried out experiments to produce the element 103 isotopes with mass numbers 255-26O [B10]. They used practically monoisotopic 249Cf and 248Cm targets which were bombarded with boron and nitrogen ions, respectively. The collection of nuclear reaction products was accomplished by using the gas jet technique. A system consisting of 28 surface-barrier detectors permitted the measurement of α spectra and half-lives at 6 stations for primary and daughter products. Special attention was paid to the problems of background due to lead impurities. The work was carried out very thoroughly and the results obtained are trustworthy though an independent confirmation has yet not taken place in all the cases. They are presented in table 2, together with the results of the first Berkeley work [B6] and with the Dubna data. It is seen that the priority of the Dubna group in the discovery of element 103 is beyond any doubt (cf. also fig. 7).
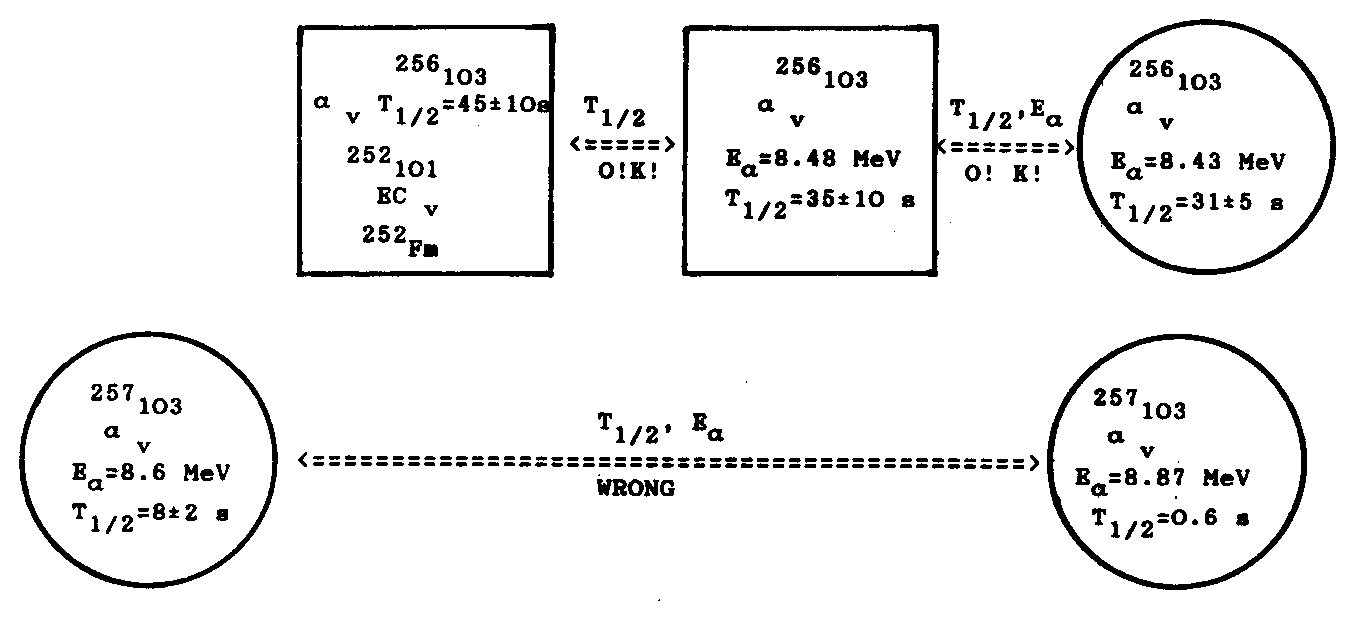
Fig. 7. The data of the works that claim the discovery of element 103. M1. G.N.Flerov et al. Doklady AN SSSR 120, 73 (1958). ZhETF, 38, 62 (1960). 2. O.Hahn, Verh. D. phys. Ges. 11, p.55, (1909). B3. A.Ghiorso, T.Sikkeland, J.R.Walton, G.T.Seaborg, Phys. Rev. Lett. 1, 18 (1958). B4. A.Ghiorso, Physics Today 20, 25 (1967). B5. A.Ghiorso, Proc. of the Second Conf. on Reactions between Complex Nuclei, Gatlinburg, May 2-4, 1960, p.195. B6. A.Ghiorso, T.Sikkeland, A.E.Larsh, R.M.Latimer, Phys. Rev. Lett. 6, 473 (1961). D7. E.D.Donets, V.A.Shchegolev, V.A.Ermakov, At. En., 19, 109 (1965). D8. G.N.Flerov et al., Nucl. Phys., A106, 476 (1967). B9. G.Seaborg, Actinides Reviews, 1, 3 (1966). B10. K.Eskola, P.Eskola, M.Nurmia, A.Ghiorso, Phys. Rev. C 4, 632 (1971). D11. E.D.Donets, V.A.Druin, V.L.Mikheev, At. En. 25, 87 (1968), Ann. Phys. 3, 331 (1968). D12. E.D.Donets, V.A.Shchegolev, V.A.Ermakov, At. En. 16, 195 (1964). D13. E.D.Donets, V.A.Shchegolev, V.A.Ermakov, Preprint JINR P-2114,Dubna, 1965, Yad. Fiz.2, 1O15 (1965). D14. V.L.Mikheev, PTE No.4, 22 (1966). D15. G.N.Flerov et al. Nucl. Phys. A106, 476 (1967). D16. V.A.Druin, Yad. Fiz.12, 268 (1970), Sov. J. Nucl. Phys. 12, 146 (1971). D17. Yu.T.Chuburkov et al. JINR 07-4085, Dubna, 1968; Radiokhimiya v.ll, 394(1969): Inorg. Nucl.Cham., 31, 3113(1969). |